A Scalable Route for the Regio- and Enantioselective Preparation of a Tetrazole Prodrug
- emiliefarrugia315
- Nov 8, 2017
- 1 min read
Updated: Aug 22, 2021
Anne Akin, Mark T. Barrila, Thomas A. Brandt, Anne-Marie Dechert-Schmitt, Pascal Dube, David D. Ford, Adam S. Kamlet, Chris Limberakis, Andrew Pearsall, David W. Piotrowski, Brian Quinn, Sarah Rothstein, Jerry Salan, Liuqing Wei, and Jun Xiao, “A Scalable Route for the Regio- and Enantioselective Preparation of a Tetrazole Prodrug: Application to the Multi-Gram-Scale Synthesis of a PCSK9 Inhibitor”, Organic Process Research & Development, 2017 21 (12), 1990-2000.
Abstract
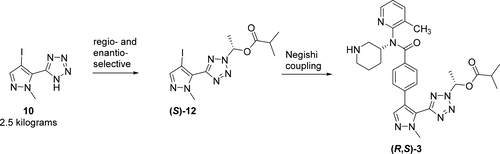
The synthesis of multigram quantities of small molecule PCSK9 inhibitor (R,S)-3 is described. The route features a safe, multikilogram method to prepare 5-(4-iodo-1-methyl-1H-pyrazol-5-yl)-2H-tetrazole (10). A three-component dynamic kinetic resolution between tetrazole 10, acetaldehyde, and isobutyric anhydride was catalyzed by a chiral DMAP catalyst to afford enantiomerically enriched hemiaminal ester (S)-12 on multikilogram scale. Magnesiation, transmetalation, and Negishi coupling provided access to Boc-intermediate (R,S)-13, which was deprotected to provide (R,S)-3 in multigram quantities.
See the full article here.



Comments