Our Services
We provide engineering services through all aspects of process development, enabling value-added solutions that scale to meet your needs.
Quality Policy & Objectives
Quality Policy
-
Nalas is dedicated to delivering high-quality products and services through a culture of continuous improvement, innovation, and rigorous adherence to industry and ISO 9001 standards, ensuring customer satisfaction and operational excellence.
-
Nalas considers its employees our most valuable asset. Our qualified and trained staff is continuously learning to ensure competence in their role and growth for their future.
-
Nalas’s management shall make available all the requisite measures and resources to achieve its quality policy and objectives defined in the Quality Manual.
Quality Objectives
-
Nalas is committed to ensuring customer satisfaction as measured by customer feedback, repeat customers or customer retention, and no customer complaints.
-
Nalas products comply with finished product specifications and agreed upon customer requirements with minimal reworks, and no defects (e.g., FOD).
-
Nalas products and services deliver on-time and on-cost to customers > 80%.
-
Nalas provides technical and team leadership opportunities through training and increased project responsibilities across 10% of the Nalas workforce.
-
Nalas embraces Continuous Improvement through monitoring, measuring, reviewing, and acting upon opportunities.
ISO 9001 Certification
Nalas is awarded ISO 9001:2015 certification, an internationally recognized standard that assures our products and services meet the needs of customers through an effective quality management system. We deliver uncompromising quality to our customers, partners, and regulatory authorities with continual improvement as our mindset.
Quality Control
At Nalas, we design our processes with quality in mind. Quality Control measures are implemented throughout the product life cycle from planning to delivery. Raw materials are sourced from qualified suppliers and released against specifications that ensure a predictable and consistent finished product. Sampling and testing strategies are designed in collaboration with R&D and Production Teams to determine critical raw material attributes. We also conduct In-Process and Finished Product testing against established specifications to assure consistent, reliable, and uniform product. Quality Control goes beyond analytical testing. Our process is structured around clearly written procedures and documentation to ensure data integrity, instrument qualification, and effective training. Nalas Quality Control assists in minimizing risk and efficiently delivering quality products.
Analytical Chemistry
Analytical Testing
Analytical testing is a fundamental component to the analytical chemistry group at Nalas. We have a wide range of techniques available include but are not limited to general titrations, density measurements, refractive index, residual water content, residual solvent content, DSC and TGA, Raman, FTIR, UV-Vis, LC, SFC/MS, LC/MS (including UPLC), GC/FID/MS, ion chromatography, GPC, accurate mass measurement, and NMR spectroscopy. These technologies and the requisite techniques are the cornerstone to our analytical capabilities here at Nalas Engineering.
Structural Elucidation
Structure elucidation is critical to any chemistry-related project. The appropriate identification and characterization of synthetic materials, impurities, metabolites, etc. provides insight into many aspects of chemical processes, whether medicinal chemistry, process chemistry or metabolism. We have state-of-the-art capabilities and the knowledge to solve a vast array of structure elucidation problems. Our capabilities allow us to elucidate chemical structures via modern analytical methodologies allowing for complete assignment for all reporting needs, whether in the scientific literature or a regulatory filing. Our team of scientists have the knowledge and capabilities to provide solutions to your structure elucidation needs.
Method Development
Chromatographic method development is a critical skill set in any analytical group performing work on small molecules. Nalas has a great deal of expertise in method development ranging from purity determining methods to methods designed for component isolation. We have a diverse array of chromatographic techniques at our disposal. These include HPLC/UPLC, SFC, GPC, IC, with a wide array of detection methods to aid in the development of the needed separation.
Flow NMR
Reaction monitoring via NMR spectroscopy provides significant insights into reaction kinetics, impurity profiles and mass balance across all NMR observable species being studied. The data is inherently quantitative during the course of the reaction. Therefore, one can collect quantitative data of all starting materials, intermediates, impurities and products over the entire course of the reaction. Here is an example of this application for the hydrolysis of acetic anhydride.

At Nalas, we provide high resolution (400 MHz) for reaction monitoring via NMR spectroscopy. Our Bruker NEO 400 MHz spectrometer is equipped with an InsightMR™ accessory for collection of real time NMR data. InsightMR™ by Bruker is a FlowNMR technology that combines hardware and software into one solution for online monitoring of chemical processes for real-time understanding and optimization.

Nalas has one of the few InsightMR™ systems in the US available to assist you with your chemistry challenges. Let Nalas help you better understand and improve your chemical reactions to give you an improved, safer and more affordable process.
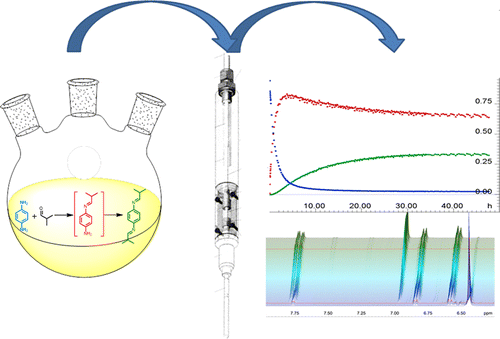
Reference: Foley, David A.; Bez, Eckhard; Codina, Anna; Colson, Kimberly L.; Fey, Michael; Krull, Robert; Piroli, Don; Zell, Mark T.; Marquez, Brian L. Analytical Chemistry, (2014), 86(24), 12008-12013.
Impurity Profiling
Impurity profiling goes hand in hand with method development and structure characterization. Being able to adequately develop a method to observe the impurities in a particular chemical transformation is an important step in understanding material balance, fate and purge and degradation. Having the ability to not only observe but to characterize the impurities via MS or potentially NMR has tremendous impact in process understanding.

Solid-State Chemistry
As experts in solid-state chemistry, Nalas offers salt, cocrystal and polymorph screening to encompass the full solid-state landscape that one may encounter in preparation for full-scale production and preparation of patent estates. Full analytical characterization of new and known solid-state forms is available to meet short development timelines and emergency in-the-tank trouble shooting. To assess the physical properties of crystalline material, advanced techniques in solid-state characterization are employed, which include Powder X-ray Diffraction (PXRD), Differential Scanning Calorimetry (DSC), Thermogravimetric Analysis (TGA), Optical Microscopy, and High Performance Liquid Chromatography (HPLC) for solubility determinations.

The study of crystallization bridges the disciplines of chemistry, materials science, and engineering. Through crystallization a collection of randomly orientated molecules in solution (or from a disordered semisolid) are assembled into a highly ordered solid-state through pair-wise molecular and atomic interactions. Characterizing the solid-state of new molecules or molecular complexes drives the fundamental understanding of their physicochemical properties and thus facilitates efficient development for bulk chemical production, fine chemical research, pharmaceutical development, and highly energetic materials development. The Nalas team will construct a crystallization process map to navigate the solid form landscape of polymorphs and solvates to isolate finished goods in high yield and purity.
Isolation by precipitation or crystallization requires fundamental knowledge of a systems kinetic and thermodynamic properties such as solubility, supersaturation, desaturation rate,
dissociation constant (pKa), and Vapor Liquid Equilibria (VLE) in order to map the solid form
landscape.




